Background:
There is a need for improved assessment of cardiac involvement in systemic amyloidosis by non-invasive methods to increase accuracy of diagnosis and to assist with monitoring and prognostication of the condition. We have previously shown that 18F-florbetaben, a novel amyloid binding radiotracer, can identify the presence of cardiac amyloid in positron emission tomography (PET/CT) imaging. We aim to evaluate the role of imaging with 18F-florbetaben PET/CT, cardiac magnetic resonance (CMR) and transthoracic echocardiography (TTE) in the initial diagnosis and response assessments of patients with light chain (AL) cardiac amyloidosis (AL-CA).
Methods:
Twelve patients diagnosed with systemic AL amyloidosis and proven or suspected cardiac involvement, between June 2018 and December 2020, were prospectively recruited to undergo 18F-florbetaben PET/CT, CMR and TTE imaging prior to commencement and 6 months post systemic therapy.
40-minute dynamic and static PET/CT images were acquired following 18F-florbetaben radiotracer injection. Left ventricular myocardial standardised uptake values (SUV) were recorded and used to graph time activity curves (TAC). Percentage myocardial tracer retention (MTR) was derived from the dynamic data. CMR was performed using a 3.0T MRI system. The Modified Look-Locker Inversion recovery (MOLLI) method was utilized to generate native and gadolinium-enhanced T1 mappings, which were then used to calculate the global extracellular volume (ECV). PET and CMR indices were compared to 2D-speckled TTE metrics and cardiac biomarkers (BNP, cTnI) using Pearson Correlation for statistical analysis. Overall survival was calculated using the Kaplan-Meir method.
Results:
Participant characteristics were as per table 1. Five deaths occurred during the study, all due to progressive AL amyloidosis; with 4 of these occurring prior to the planned second imaging timepoint. Two-year survival was 58.3%, with a mean overall survival of 7.3 years (95% CI 4.08 - 10.52).
All patients had a baseline PET/CT; 8 were able to have a concurrent CMR. The remaining 4 were unable have a CMR due to incompatible devices or were clinically unwell at the time. Seven patients went on to have a repeat PET/CT and 5 had comparative CMR imaging 6 months after commencing treatment.
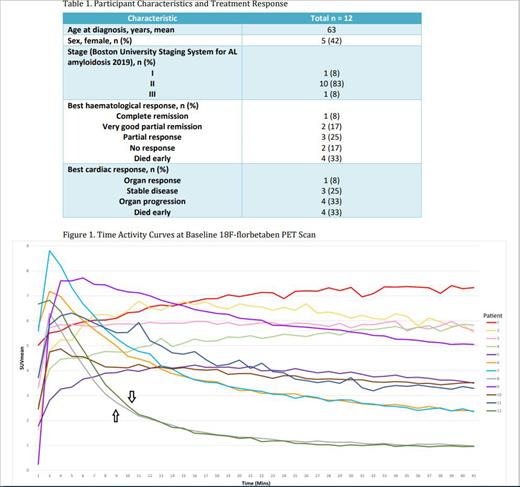
Baseline 18F-florbetaben PET/CT scans demonstrated characteristic AL-CA patterns of prolonged myocardial tracer retention TAC in 10 out of the 12 patients (Figure 1). Patients' 8 and 12 TACs (highlighted with arrows in Figure 1) did not follow the usual AL-CA MTR pattern and their MTRs were calculated to be below a previously defined threshold of ≥40% for the presence of cardiac amyloid. This prompted review of the diagnosis of AL-CA in these patients and both were found to have alternative causes for the TTE and cardiac biomarker abnormalities which were initially interpreted as AL-CA.
Analysis of the different imaging metrics showed significant correlation between MTR and ECV (r=0.575; p=0.04) as well as ECV and GLS (r=0.62; p=0.024). ECV also correlated with BNP (r=0.637; p=0.019) as did GLS and BNP (r=0.678; p=0.002). There was no association between MTR and cardiac biomarkers.
8 patients were assessable at the 6-month timepoint; only 1 patient had a cardiac response at 6 months by the International Society of Amyloidosis (ISA) criteria and they demonstrated a >10% reduction in MTR on follow-up PET/CT. Two patients had stable cardiac disease as per ISA criteria: 1 had >10% reduction in MTR and went on to achieve subsequent cardiac response without further therapy; the other had an increase in MTR by 18.5% and went on to salvage ASCT for suboptimal haematological and cardiac responses (partial remission and stable organ disease). Four patients had cardiac progression by ISA criteria with a worsening of MTR by at least 15% (mean=19.1%).
Conclusion:
Patients with AL-CA display prolonged 18F-florbetaben retention on PET/CT imaging which also appears useful to exclude the presence of cardiac amyloidosis in patients with co-existent cardiac conditions. Preliminary data supports the role of 18F-florbetaben PET/CT in cardiac response assessment, but further studies are required to determine the optimal timing of follow up imaging, which is likely to be beyond the 6-month time point, and to determine clinically significant MTR ranges for monitoring and prognostication.
Disclosures
Mollee:Pfizer: Research Funding; Cilag: Research Funding; Janssen: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal